Testimonials

Testimonial from Siemens Healthineers
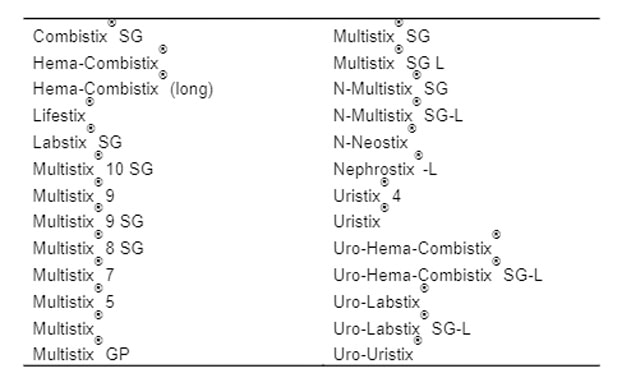
Urinalysis Quality Controls for Siemens Urinalysis Strips with ID Band
This bulletin provides important information about the use of urinalysis controls with Siemens urinalysis strips having ID bands. If you use any of the test strips listed in the Appendix, please follow the recommendations in this bulletin to optimize your testing process. The urine test strips listed in the Appendix have been updated to include a new identification (ID) band. The ID band has been added near the test strip handle and appears as a white area on the strip, as shown in the figure below.
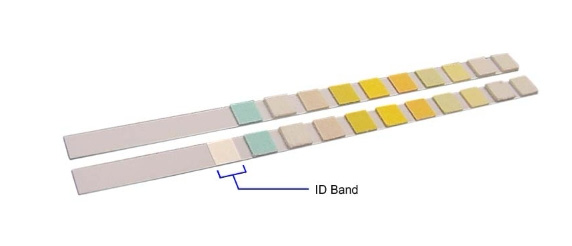
The ID bands will enable important automatic checks (Auto-Checks) when used with the following Siemens urinalysis analyzers:
- CLINITEK Status®+ Analyzer
- CLINITEK Status® Analyzer (upgraded with software version 2.000 or later)
To learn more about these features, talk to your Siemens representative or visit www.medical.siemens.com.
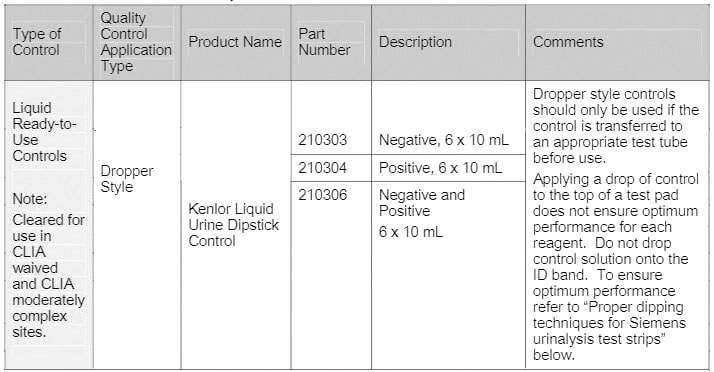
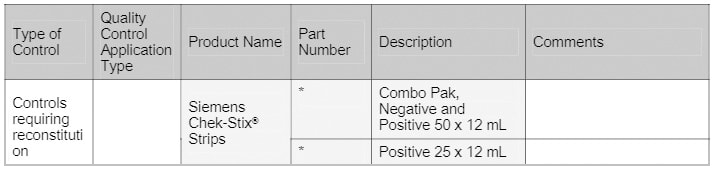
* Contact your Siemens representative for product codes
* For questions and comments regarding our products, or to place an order, please fill out the adjoining form.
Note This information does not apply to CLINITEK Atlas® or CLINITEK AUWi™ System users.
Proper Dipping Technique for Siemens Urinalysis Strips
Proper Dipping Technique for Siemens Urinalysis Strips
Handling of the urine test strips with the new ID bands has not changed. Be sure to continue to use the proper handling technique stated in the product labeling to optimize test processing.
- Do not touch any of the test pads, including the ID bands.
- Proper dipping technique is to dip the test pads fully in the sample and then drag the edge of the strip against the container rim to remove excess solution. Blot the edge of the strip when using CLINITEK 50 and CLINITEK Status analyzers.
Note The ID band can be dipped if proper dipping technique is used to remove excess solution.
Important Update
The following quality control materials have been shown to cause errors on CLINITEK analyzers when used with urinalysis test strips listed in the Appendix.
- NERL Sentry Urine Dipstick Control
- Bio-Rad Liquichek™ Urinalysis Control
- MAS® Urinalysis Control
- HYCOR KOVA Liqua-Trol™
Trademark Information
CLINITEK, CLINITEK Advantus, Atlas, and AUWi are trademarks of Siemens Healthcare Diagnostics. Chek-Stix , Combistix, Lifestix, Multistix, Neostix, Nephrostix, Uristix, and Labstix are trademarks of Bayer HealthCare and are licensed for use by Siemens Healthcare Diagnostics qUAntify and Liquichek are registered trademarks of Bio-Rad Laboratories Dipper is a trademark of Quantimetrix Corporation MAS is a trademark of Microgenics (ThermoFisher Scientific) KOVA and Liqui-Trol are trademarks of HYCOR Biomedical, Inc
Appendix